Ingestible balloon
The Endoscopy and Digestive Function Testing Unit is a pioneer in the treatment of the ingestible intragastric balloon, a device that helps patients with a Body Mass Index (BMI) of 27 or above to lose weight.
What is an ingestible balloon?
The Elipse ingestible balloon is a device that can help overweight and moderately obese patients lose weight. This is an implant designed to be swallowed directly by the patient. In this way, the balloon is swallowed by the patient in a capsule and, once in the stomach, it fills with a solution until it reaches a volume of 550–600 cubic centimetres.
While the intragastric balloon is inserted into the patient's stomach using endoscopy, in the case of the ingestible balloon, the patient can swallow the capsule directly.
The capsule, as in the case of the intragastric balloon, will float freely in the gastric cavity.
Does the placement process require endoscopy?

No. This technique does not require endoscopy but must be performed under medical supervision.
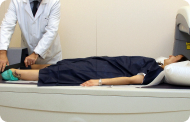
The balloon is designed to be swallowed by the patient so that it remains in the stomach. Although placement does not require endoscopy, it does require radioscopy to verify the correct placement of the device in the stomach before inflating the balloon.
How is it placed?
The patient drinks a glass of water and swallows the capsule catheter containing the balloon.
Once the patient swallows the capsule and it reaches the stomach, it opens, fills up and releases the balloon with a content of 550–600 cubic centimetres of sterile liquid.
Once inflated, the doctor will slowly remove the catheter from the patient's mouth and the balloon will remain ‘floating’ in the stomach.
The simplicity of the placement technique means that it can be performed on an outpatient basis and does not require sedation, so that the patient, after verification of the placement of the balloon in the gastric cavity, can return to their daily activities.
When is the ingestible balloon indicated?
The use of an ingestible balloon is recommended for patients between the ages of 18 and 60 who are overweight or moderately obese and who have been unable to lose weight permanently with other programmes. It is also indicated for use in obese patients who wish to lose weight before surgery in order to reduce associated risks.
What is the ingestible intragastric balloon used for?
The balloon, by partially filling the stomach, reduces the feeling of hunger. This sensation helps patients stick to their diet and change their eating habits, which they must follow under medical supervision after the device is fitted.
What will determine the volume of stomach occupancy?
The volume of space occupied in the stomach will depend on the degree of excess weight and the size of the patient's stomach.
How big can an ingestible balloon get?

An ingestible balloon can reach a volume of 550–600 cubic centimetres.
How does the device work?
The balloon produces a distinct feeling of fullness and, consequently, a lack of appetite. These effects help patients modify their eating habits by following a proper diet that allows them to lose weight. It is important to change the patient's eating habits and exercise to maintain weight loss in the months following treatment.
What are the steps after ingesting the balloon?
After ingesting the balloon, the patient must undergo multidisciplinary medical follow-up, including endocrine care to monitor aspects such as hypertension and diabetes. It also requires the development of a dietary programme to help modify the patient's eating habits.
What is the secret to this technique's success?
The balloon itself does not cause weight loss. The patient's behaviour is very important for the success of this treatment, and they must comply with the follow-up programme.
The patient should continue to be monitored by a specialist who will supervise their diet and teach them healthy eating habits.
What are the risks associated with this device?
The risks are minimal, although placement requires specific medical supervision.
Will the patient feel any discomfort after the balloon is inserted?
The patient may feel some discomfort during the first few days, until their stomach gets used to the balloon. The possible symptoms include diarrhoea, vomiting and nausea.
What precautions should be taken after the balloon is inserted?
Hydration guidelines in the first few days after this device is fitted should follow specific criteria, such as drinking only a few sips of water on the first day and trying to drink much more water in the following days. During this time, it is important to avoid eating solid foods.
The specialist will inform you about the procedure to follow and will prescribe a specific diet.
How long can it stay in the stomach?
The device must remain in the stomach for around four months.
How is the ingestible balloon removed from the body?
The Elipse ingestible balloon remains in the stomach for four months. After this time, the balloon spontaneously disintegrates and can be safely removed as the silicone dissolves. No endoscopy is required for removal.
What are the contraindications for the ingestible balloon?
It may vary in each patient, but as with any other medical approach, adverse and unexpected reactions may arise from the drugs, materials or techniques used.
During the first three days after the device is fitted, the patient may experience nausea, cramps or vomiting.
How much weight can you lose with this technique?
Studies conducted prior to CE certification conclude that, four months after treatment, losses exceed 50% of excess weight once treatment has been completed and the dietary habit change programme has been followed in parallel with the time the balloon remains in the stomach.
Likewise, results will depend largely on how patients adopt and maintain long-term lifestyle changes, both in terms of eating habits and physical exercise.
Is it a validated method?
Yes, the use of the ingestible balloon is certified and approved by the EC.