Tissue Regenerative Therapy Institute (ITRT)
ITRT develops advanced therapy medicinal products based on Cultured Mesenchymal Cells (CMC), manufactured in GMP-compliant cleanrooms, with personalized cell dosing and evaluated in authorized clinical trials.


 Tendon injuries
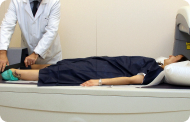
Tendon injuriesCMC administered intratendinously has demonstrated structural regeneration in the patellar, Achilles, hamstring, and supraspinatus tendons, validated by MRI and associated with sustained functional improvement.
 Intervertebral disc injuries
Intervertebral disc injuriesIn lumbar disc degeneration, intradiscal administration of CMC has shown structural recovery on MRI and sustained pain reduction, with extended clinical follow-up.
 Cartilage injuries
Cartilage injuriesITRT has evaluated CMC in osteoarthritis of the knee, hip, shoulder, and other peripheral joints, whether degenerative or post-traumatic, with clinical evidence of cartilage regeneration and functional improvement.
ITRT is a leader in advanced cell therapy medicines using Cultured Mesenchymal Cells (CMC), applied in a personalised manner and with individual authorisation from the AEMPS. We guarantee traceability, safety and differentiation from unauthorised practices.
 Pioneers in regulated cell therapyOver 20 years developing advanced therapy medicinal products with CMCs within the framework of authorized clinical trials.
Pioneers in regulated cell therapyOver 20 years developing advanced therapy medicinal products with CMCs within the framework of authorized clinical trials. Personalized and authorized medicinal productEach administration requires medical evaluation, personalized cell dosing, and individual authorization from the AEMPS.
Personalized and authorized medicinal productEach administration requires medical evaluation, personalized cell dosing, and individual authorization from the AEMPS. Regulatory assuranceWe strictly adhere to Spanish and European regulations regarding GMP manufacturing, authorized clinical application, and quality control.
Regulatory assuranceWe strictly adhere to Spanish and European regulations regarding GMP manufacturing, authorized clinical application, and quality control. Clinical safety and traceabilityManufacturing in GMP-certified cleanrooms, with cell characterization, quality controls, and structural and functional clinical monitoring.
Clinical safety and traceabilityManufacturing in GMP-certified cleanrooms, with cell characterization, quality controls, and structural and functional clinical monitoring. Commitment to scientific legalityWe stand apart from unauthorized practices lacking regulatory support or validated clinical evidence.
Commitment to scientific legalityWe stand apart from unauthorized practices lacking regulatory support or validated clinical evidence.
Health and Wellbeing Blog
- Experts in regulated and personalized cell therapiesDr. Robert Soler, CEO and Medical Director, is a leading figure in the development of advanced therapy medicinal products with Cultured Mesenchymal Cells (CMC). At ITRT, a specialized clinical and scientific team combines medical excellence, operational quality, and regulatory compliance across every stage of the therapeutic process.
- Over 20 years of clinical research with Cultured Mesenchymal Cells (CMC)Since 2002, ITRT has developed a pioneering and regulated clinical model.
ITRT is a medical institute specialized in the development of advanced therapy medicinal products, created from cells obtained either from the patient or a donor, and cultured under controlled conditions for clinical use.
All procedures at ITRT are backed by authorized clinical trials and published scientific evidence. It operates under a fully regulated model, with cleanroom GMP manufacturing, individual authorization by the AEMPS, and full traceability from cell collection to clinical follow-up.
ITRT also has a cryopreservation system that allows CMCs to be stored under optimal conditions for later use, ensuring product stability and control for mid- or long-term planned treatments.
It is a medicinal product regulated by the Spanish Agency of Medicines and Medical Devices (AEMPS) and the European Medicines Agency (EMA), including cell-based, gene, or tissue-engineered therapies.
In the case of ITRT, these are advanced therapy medicinal products developed from Cultured Mesenchymal Cells (CMC), manufactured under GMP (Good Manufacturing Practice) standards in certified cleanrooms, and authorized individually for each patient.
They are progenitor cells derived from bone marrow, isolated and expanded over 21 days in controlled culture conditions. These cells are characterized, validated, and prepared for clinical use as advanced therapy medicinal products, with quality control and traceability in accordance with GMP standards.
CMC are cell-based medicines authorized by the AEMPS, with clinical evidence, GMP manufacturing, and regulated application.
In contrast, the term "stem cells" is ambiguous, unregulated, and often used to promote practices lacking legal and scientific basis. ITRT explicitly rejects this term due to its imprecision and use outside the authorized medical framework.
The safety and efficacy of ITRT’s medicinal products are supported by AEMPS-approved clinical trials, preclinical studies, scientific publications, and long-term clinical follow-up.
CMC developed by ITRT have been evaluated in authorized clinical trials for tendinopathies, osteoarthritis, discopathies, pseudoarthrosis, osteonecrosis, and xerostomia, showing evidence of structural regeneration and functional improvement.
Eligibility can only be determined following an individualized medical evaluation. The decision depends on specific clinical factors, imaging diagnosis, and medical history. Not all patients are candidates, and selection must be made by a specialized team.
Depending on the diagnosis, the medicine may be administered in a single dose or through a series of applications. The route of administration is determined based on the affected tissue and specific medical recommendation.
In the case of the medicinal products developed by ITRT, they are applied locally through minimally invasive procedures: intra-articular, intratendinous, intradiscal, or subchondral infiltration, depending on the clinical indication and authorized protocol.
Depending on the clinical indication and authorized protocol, autologous cells (from the same patient), allogeneic cells (from a compatible donor), or cryopreserved cells (previously stored at ultra-low temperatures) may be used. All cell types undergo quality control, cell characterization, and require individual authorization from AEMPS prior to clinical use.
AEMPS (Spanish Agency of Medicines and Medical Devices) is the authority responsible for individually authorizing each product, overseeing its manufacturing, ensuring safety, and controlling its clinical use in compliance with the current legal framework.
Yes, as long as there is a justified medical indication and the regulated procedure for individual authorization by AEMPS is followed. Access is restricted to medical teams with prior experience using the medicine in authorized clinical trials and must comply with all clinical, technical, and ethical criteria.
All ITRT medicinal products meet the quality controls required by GMP standards: sterility, viability, identity, potency, and absence of contaminants. In addition, structural and functional clinical follow-up is conducted over several years.
The entire process of medical evaluation, GMP manufacturing, and AEMPS authorization can be completed in approximately one week, depending on the cell type used and clinical logistics.
Treatment includes not only the administration of the medicine but also individualized clinical follow-up, which may extend from 2 to 5 years depending on the pathology. During this time, periodic structural and functional evaluations are performed, including imaging tests such as MRI, to assess clinical progress, stability of biological effects, and long-term safety.
ITRT develops only authorized medicinal products, with full traceability, clinical evidence, quality control, and full EMA/AEMPS regulatory compliance.
Unlike other unregulated offers, ITRT provides personalized, safe, and legally supervised medicine, explicitly rejecting the use of unauthorized products or misleading terms such as "stem cells."
Our team is at your complete disposal.
![]() Centro Médico Teknon
Centro Médico Teknon
Carrer de Vilana, 12, 08022 Barcelona
![]() Opening hours
Opening hours
Open 24 hours
![]() Phone Numbers
Phone Numbers
932 906 200
900 301 013